In Copyright Since September 11, 2000. This web site isn't affiliated with, and doesn't represent, any Kaiser entity including but not limited to Permanente. This is instead a public interest nominative use site. Permission is granted to mirror this web site under Creative Commons CC BY-NC-SA 4.0. Please acknowledge where material was obtained.
Link for Translation of the Kaiser Papers | ABOUT US | CONTACT | MCRC | Kaiser Diagnostic and Treatment Documents
CLINICAL
PRACTICE GUIDELINE for
MANAGEMENT of DIABETES MELLITUS
 PRACTICE
GUIDELINES
PRACTICE
GUIDELINES
GUIDELINE ISSUED: APRIL 1998 UPDATE: FEBRUARY 2000
CLINICAL PRACTICE GUIDELINE for MANAGEMENT of DIABETES MELLITUS
ENDORSED BY: CHIEFS OF ENDOCRINOLOGY CHIEFS OF MEDICINE
INTRODUCTION This is an update of the Clinical Practice Guidelines for the Management of Diabetes Mellitus intended to incorporate the results of clinical trials that have been published since the April 1998 release of the guideline.
Additional issues important to the management of diabetes mellitus are thoroughly discussed in the April 1998 guideline. These include: management of type I diabetes, patient education and behavior change, psychosocial issues, nutrition, physical activity, aspirin prophylaxis, lower extremity complications, adverse outcomes of pregnancy, and psychiatric complications. For more detailed information see the Kaiser Permanente Northern California Clinical Practice Guidelines for the management of Diabetes Mellitus, April 1998.
Key points
* Glycemic control, regardless of the way it is achieved, will reduce microvascular complications in patients with type 2 diabetes. The therapeutic goal for glycemic control is a HbAlc of < 7.0%. *Monotherapy and combination drug therapies have changed (both standard and optional therapies). * Blood pressure (BP) control reduces macrovascular and microvascular morbidity and mortality. The target blood pressure should be < 130/85 mm Hg for all patients with diabetes mellitus. If there are early signs of nephropathy, the patient with diabetes should have a target BP of 125/75 mm Hg or lower, if tolerated. * Hyperlipidemia is a common comorbidity associated with diabetes. For a given level of cholesterol, patients with diabetes have a much greater frequency of cardiovascular events; therefore, aggressive therapy of diabetic dyslipidemia is indicated. Goals: LDL < 100 mg/dL with vascular disease; LDL < 130 mg/dL no vascular disease: HDL > 50 mg/dL; triglycerides < 200 mg/dL.
SCREENING & DIAGNOSIS
A fasting plasma glucose (no food or beverage for at least 8 hours prior to the test) remains the test of choice for diagnosing diabetes mellitus. While glycosylated hemoglobin (HbAlc) has proven to be a very valuable tool for monitoring treatment, further studies continue to discourage its use for screening or diagnosis. This is due to both the lack of standardization as well as poor reproducibility in healthy adult populations. One meta-analysis concluded that a HbAlc level of 7 % or higher (or > 1 % above upper limit assay) has sufficiently high sensitivity for identifying diabetes that requires treatment. Unfortunately, at this level, cases of impaired fasting glucose (IFG) or impaired glucose tolerance (IGT), as well as some cases of diabetes will be missed.
The prevalence of IGT and type 2 diabetes in women with polycystic ovarian syndrome (PCOS) is substantially higher than expected when compared with age and weight matched populations of women without PCOS. There is also a higher conversion from IGT or IFG to type 2 diabetes each year. It is estimated that 35% of PCOS patients have IGT, and 10% will be diagnosed with diabetes before reaching the fourth decade. Therefore, this group of women should be included as a high-risk group that is appropriate for diabetes screening. Blood pressure and lipids should be aggressively managed in this group.
Recommendations
* Screening for type I diabetes, outside of a research environment, is not recommended. Community screening in the general population for type 2 diabetes is not recommended due to its low yield. * Screening of high-risk groups for type 2 diabetes is reasonable based on current evidence. The major risk factors for type 2 diabetes are <> Obesity (>120% of desired body weight or a BMI >27 kg/m) 0 <>A first degree relative with diabetes <> African-American, Hispanic, Native American, Pacific Islander <> Delivery of a baby > 9 pounds or a previousdiagnosis of gestational diabetes <> Hypertension (³140/90 mm Hg) <> HDL-C £ 35 mg/dL or a triglyceride >250mg/dL <> History of impaired glucose tolerance (IGT) or impaired fasting glucose (IFG) <> Women with polycystic ovarian syndrome (PCOS)
The available data are currently insufficient to make an evidence-based recommendation concerning optimal screening intervals among high-risk groups; however, it is the consensus of the Diabetes Guidelines Team that annual screening by fasting blood glucose level of patients with a history of gestational diabetes, IGT, and PCOS should be done.
For additional information on screening and diagnosis, see pages 5-6 in the Kaiser Permanente Northern California Clinical Practice Guidelines for the Management of Diabetes Mellitus, April 1998.
MANAGEMENT OF TYPE 2 DIABETES Glycemic Control
The United Kingdom Prospective Diabetes Study (UKPDS) evaluated 3867 patients with newly diagnosed type 2 diabetes over 10 years. Tighter glucose control with insulin, metformin or sulfonylureas was compared to conventional treatment. Average HbAlc over 10 years was 7% in the intensively treated group and 7.9% in the conventionally treated group. Despite this minimal difference, there were fewer microvascular complications and a trend towards reduced macrovascular diabetes complications in the intensively treated patients.
HIGHLIGHTS OF THE UKPDS *Type 2 diabetes is progressive and additional oral medication and/or insulin are likely needed to maintain adequate glycemic control.
*No advantage or disadvantage was observed with any particular agent in attaining glycemic control.
* Obese patients taking metformin had fewer diabetes-related complications along with less weight gain and fewer hypoglycemic episodes. Mortality and stroke in these obese patients were decreased in the intensive therapy group who received metformin. For these reasons, it is the consensus of the Diabetes Guideline Team that metformin is the drug of choice in most overweight type 2 diabetes patients without contraindications (e.g., heart failure, renal or hepatic insufficiency, or hypoxic states).
* Even small decreases in average blood glucose resulted in decreased microvascular complications.
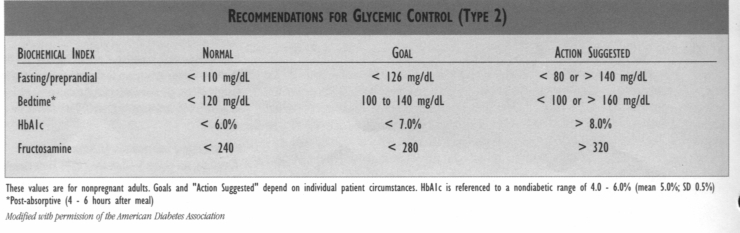
GLYCEMIC CONTROL MEASURES The recommendation of the Diabetes Guideline Team is that the therapeutic goal for glycemic control is < 7.0% (see table below) based on the results of the UKPDS and the recommendation of the American Diabetes Association. However, at this point, this value differs from the goals set for glycemic control using the Quality Indicators for the APC Population Management Diabetes Management Program and for HEDIS. The Quality Indicators for the APC Diabetes Management Program measures good control as HbAlc < 8.0 and fair control as HbAlc < 10.0% (in 2001, HbAlc < 9.5%). In contrast, HEDIS does not have a measure for good glycemic control, but does set a measure for poorly controlled diabetes as HbAlc of >9.5%.
CLINICAL CONSIDERATIONS * After a new drug is initiated or when the dosage of a medication is adjusted, follow up (e.g., by telephone appointment visit [TAV]) with the patient. Ask about glycemic control and hvpoglycemic symptoms.
* Consider one of the following if glycemic control is suboptimal or unstable before concluding that drug therapy has failed: <> Occult infections (e.g., urinary tract infections) <> New onset endocrinopathy (e.g., hyper- or hypothyroidism, Cushing's Syndrome, adrenal insufficiency) <> Overeating to compensate for hypoglycemia <> Dosing intervals and dosages may need adjusting regularly, using self-monitoring of blood glucose as well as symptom monitoring.
* When adding an insulin sensitizer (e.g., metformin or thiazolidinediones), watch for hypoglycemia. Reducing the dose of insulin or sulfonylurea is a desired effect of adding the insulin sensitizers. Warning patients of hypoglycemia and having an action plan can improve compliance.
SELF-MONITORING OF BLOOD GLUCOSE (SMBG) Frequency and timing of SMBG in Type I and Type 2 patients should be a collaborative decision between the provider and the patient, based on the patient's individual needs, intensity of management and goals of treatment plan to help patient adjust therapy.
The frequency of testing should be adjusted to both motivate patients and enhance their glycemic control and lifestyle. Patients who do not adjust their insulin dosage based on their blood glucose readings may not benefit from as frequent testing.
Suggested minimal frequency and timing of SMBG in diabetes can be viewed online in the Regional Diabetes Mellitus CPG section on the Kaiser Permanente Northern California intranet website at http://cl.kp.org
IMMUNIZATIONS
INFLUENZA - all patients with
diabetes should be immunized
for influenza annually, if no
contraindications exist.
PNEUMOCOCCAL VACCINE - current
CDC recommendations are
*All
people with
diabetes above the age of two
should be immunized with pneumococcal
vaccine.
* Revaccinate patients
³
65 years of age, if the person received his/her first vaccination
before age 65 and if more than
5 years have elapsed since
the first dose.
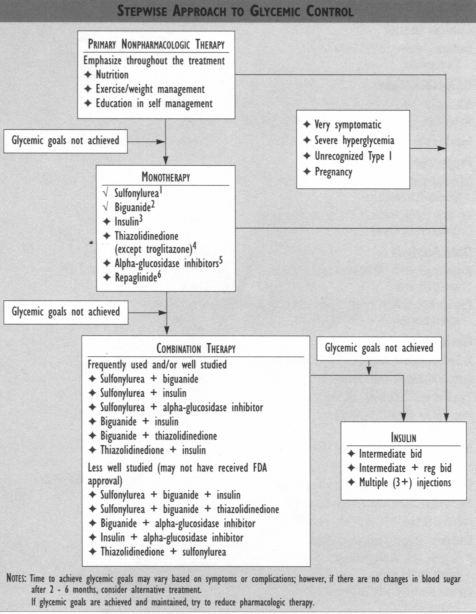
MEDICATION FOOTNOTES 'Sulfonylureas Sulfonylurcas remain a cost effective and successful treatment for many Type 2 diabetes patients. The UKPDS has alienated concerns about long-term cardiovascular safety. Sulfonylureas have been used in combination therapies with synergistic effects on blood glucose lowering.
2Biguanides Metformin (Glucophage®) continues to be the only drug in this class currently available in the United States. A subgroup of overweight diabetes patients in the UKPDS treated with metformin had fewer macrovascular complications, and its use was associated with less weight gain and fewer hypoglycemic attacks. For these reasons, it is the consensus of the Diabetes Guideline Team that metformin is the drug of choice in most overweight Type 2 diabetes patients without contraindications (e.g., heart failure, renal or hepatic insufficient or hypoxic states).
3Insulin Protocols for insulin adiustment can be viewed online in the Regional Diabetes Mellitus CPG section on the Kaiser Permanente Northern California intranet website at http://cl.kp.org
Lispro insulin (Humalog®) is designed for very rapid release and shorter duration of action. This insulin can be useful in multi-dose regimens to provide more intensive control of blood sugar with less hypoglycemia.
4Thiazolidinediones Two new medications in this class have recently been released in the United States - rosiglitazone (Avandia®, non-formulary) and pioglitazone (Actos®). Both appear to have a better safety profile than troglitazone (Rezulin®, non-formulary), with similar effectiveness. The new medications currently require bimonthly monitoring of liver function for the first year, and periodic monitoring thereafter. Rosiglitazone is dosed at 4 and 8 mg daily, while pioglitazone is dosed at 15,30, and 43 mg daily. It may take 4 - 6 weeks before a glycemic effect is seen. Expect some weight gain and volume expansion with these medications. Allow one week "washout" when switching from troglitazone to the newer medications.
5Alpha-Glucosidase Inhibitors Two preparations are currently available in the United States - acarbose (Precose®, non- formulary) and miglitol (Glyset®). The two medications are similar in action, effectiveness, and side effects. However, miglitol has near-complete absorption after oral doses, and is excreted unchanged in the urine. In clinical trials, miglitol was not associated with elevations in serum transaminase levels. Unlike acarbose, routine monitoring of liver enzymes is not recommended with miglitol therapy. Although there has been some suggestion for avoidance of miglitol in renal insufficiency, no systemic toxicity has been demonstrated. However, avoid using miglitol or acarbose if serum creatinine > 2.0 mg/dL. There are more drug interactions with miglitol than with acarbose. The consensus of the Guideline Team is that these drugs should not be used as first-line therapy. They should be considered for the subgroup of patients with high postprandial glucose levels.
6Repaglinide (non-formulary) Repaglinide (Prandin®) lowers blood glucose levels by stimulating the release of insulin from the pancreas in patients with Type 2 diabetes. Repaglinide is taken before meals to lower the postprandial increase in blood glucose. The consensus of the Guideline Team is that this drug should not to be used as first line therapy. It should be considered for the subgroup of patients with high postprandial glucose levels or those who have genuine sulfonylurea allergies.
Medication tables can be viewed online in the Regional Diabetes Mellitus CPG section on the Kaiser Permanente Northern California intranet website at http://cl.kp.org
PREVENTION AND TREATMENT OF COMPLICATIONS Beneficial Effects of ACE Inhibitors ACE inhibitors decrease mortality in post-MI patients and heart failure patients. Retinopathy and nephropathy may be delayed. Recent evidence in the HOPE study suggests that the ACE inhibitor, ramipril, has beneficial effects on cardiovascular endpoints in patients with diabetes who are over the age of 55. Current evidence is still insufficient to recommend universal use of these agents in patients with diabetes.
Hypertension Blood pressure control reduces macrovascular and microvascular morbidity and mortality in patients with diabetes.
Hypertension markedly increases the risk of macrovascular complications (MI, stroke, and peripheral vascular disease) and microvascular complications (retinopathy and nephropathy) in patients with diabetes. Prompt and continuous control of blood pressure decreases the risk of these complications. The target blood pressure should be < 130/85 mm Hg for all patients with diabetes mellitus. If there are early signs of nephropathy (albumin/creatinine ratio > 30 mcg/mg Cr), the patient with diabetes should have a target BP of 125/75 mm Hg or lower, if tolerated.
In the Hypertension Optimal Treatment (HOT) study, patients were randomized to one of three groups (diastolic BP <80 or <85or<90mmHg). A long-acting calcium channel blocker was used as initial therapy: beta-blockers, ACE inhibitors and diuretics were added as needed. Cardiovascular events and mortality were decreased with lower blood pressure in patients with diabetes, with further improvements in patients with target diastolic blood pressure < 80 mm Hg. Decreases in cardiovascular events and mortality were proportional to reductions in diastolic blood pressure.
The United Kingdom Prospective Diabetes Study (UKPDS) showed that tight control of BP (144/82) with either captopril or atenolol reduced the risk of heart failure by 56%, stroke by 44%, and death from diabetes by 32% compared to patients with BP 154/87. The risk of renal damage and progression of retinopathy also was decreased by improved BP control. Previous studies (SHEP and HDS) had shown improvements in outcome with diuretics as first-line treatment.
With reduction of macrovascular and microvascular complications of Type 2 diabetes as the goal, it appears that lowering the blood pressure is more important than the choice of antihypertensive medication used. For additional information on the treatment of hypertension in patients with diabetes, see pages 28 - 29 in the Kaiser Permanente Northern California Clinical Practice Guidelines for the Management of Diabetes Mellitus, April 1998 and page 5 in the Kaiser Permanente Northern California Clinical Practice Guideline for Screening, Evaluation and Management of Adult Hypertension, January-1999.
Lipids Hyperlipidemia is a common comorbidity associated with diabetes. For a given level of cholesterol, patients with diabetes have a much greater frequency of cardiovascular events: therefore, aggressive therapy of diabetic dyslipidemia is indicated. A common abnormal lipid pattern in Type 2 diabetes is increased triglyceride/very-low-density lipoprotein (VLDL), decreased HDL, and an LDL fraction that contains a greater proportion of small, dense atherogenic LDL particles. Initial therapy for all diabetes patients, regardless of lipid levels, should include a low-fat, low-cholesterol diet, regular physical activity, and optimal glycemic control.
Initial drug therapy for the majority of people with diabetes and hyperlipidemia is a HMG CoA reductase inhibitor [e.g., lovastatin (Mevacor®), simvastatin (Zocor®)]. Higher doses or combination therapy may be needed to achieve goals. With these changes, additional monitoring for side effects (e.g., rhabdomyolysis) is needed.
Drug
therapy should be directed first at lowering
LDL.
For diabetes patients with pre-
existing vascular disease, the
goal is to
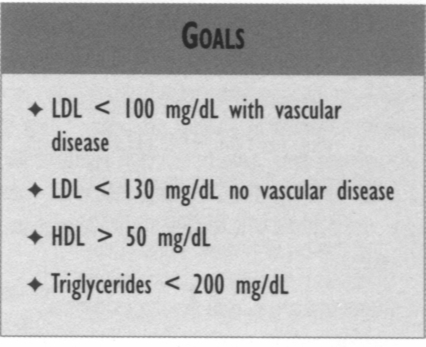
reduceLDL to < 100 mg/dL. For diabetes patients without known vascular disease, the goal for LDL is < 130 mg/dL. Some experts recommend that all patients with diabetes have an LDL < 100 because of their risk for increased cardiovascular events, especially those with additional risk factors such as tobacco use, hypertension, or microalbuminuria. Further reductions of LDL below 100 lead to reduction of atherosclerotic plaque. Isolated low HDLs (< 50) also should be treated aggressively.
For additional information on the treatment of hyperlipidemia in diabetes, see pages 30 - 31 in the Kaiser Pemianente Northern California Clinical Practice Guidelines for the Management of Diabetes Mellitus, April 1998. and page 12 in the Kaiser Permanente Northern California Clinical Practice Guidelines for Adult Cholesterol Management, November 1998.
Peripheral Neuropathy Control of pain in diabetic peripheral neuropathy continues to be challenging. A recent randomized control trial showed that gabapenlin (Neurontin®) (titrated from 900 to 3600 mg/day or maximum tolerated dosage) appeared to be efficacious. Limiting side effects included dizziness, somnolence, abdominal pain, and memory loss. Gabapentin is not FDA approved for this use.
A separate Veterans Administration Hospital study concluded that gabapentin may be an alternative for treating diabetic peripheral neuropathy pain, yet does not appear to offer considerable advantage over amitriptyline and is more expensive.
Autonomic Neuropathy GASTROINTESTINAL MANIFESTATIONS Dietary modifications include reductions in fat (<40 gm) and fiber. Four to six small meals are recommended. The use of agents that may slow gastric emptying such as calcium channel blockers, tricyclic antidepressants, anticholinergic agents should be minimized. Optimization of glucose control will further improve gastric motility.
Prokinetic agents include erythromycin, metoclopromide, or cisapride. Intravenous metoclopramide (Reglan®) 10 mg IV qid for up to 10 days or erythromycin (3 mg/kg IV q8 hours) may be used for patients who cannot tolerate oral medications.
Cisapride (Propulsid®) 10-20 mg po may be used 30 minutes before each meal and at bedtime. Important changes have been made in the cisapride labeling. These changes include recommendations for performing diagnostic tests prior to any use of cisapride. There have been continuing reports of heart rhythm disorders and deaths associated mostly in people who are either taking certain other medications or who have certain underlying conditions that are known risk factors. Review the new information prior to starting cisapride. Domperidone remains investigational.
MALE IMPOTENCESildenafil (Viagra®), a type 5 phosphodiesterase inhibitor, is effective in 50-60% of diabetic males with erectile dysfunction. The medication should be ingested approximately one hour before intended intercourse. The most common side effects include headache, flushing, rhinitis, dyspepsia and altered vision. Side effects are typically mild and transient. Sildenafil is absolutely contraindicated for men receiving any forms of nitrates. Relative contraindications include patients with CVA, MI, or life threatening arrhythmia within the prior six months, resting hypotension (< 90/50), hypertension (> 170/110), CHF, unstable angina, or retinitis pigmentosa. Patients who are physically inactive with multiple cardiovascular risk factors may be appropriate candidates for non invasive CAD screening prior to prescribing sildenafil.
A starting dose of 50 mg is appropriate for most patients and can be increased to 100 mg if the drug is ineffective. However, patients using inhibitors of P450 cytochrome system (cimetidine, erythromycin, ketoconazole, itraconazole) or protease inhibitors should begin with a 25 mg dose. Likewise, patients older than 65 years or with hepatic or severe renal disease should begin with the 25 mg dose.
Because of comparable or superior efficacy and ease of use, sildenafil can be considered the pharmacologic treatment of choice for patients with erectile dysfunction.
For additional information on peripheral and autonomic neuropathies, see pages 34 - 37 in the KP Northern California Clinical Practice Guidelines for the Management of Diabetes Mellitus, April 1998.
Nephropathy There are adequate data that ACE inhibitors, calcium channel blockers, and angiotensin- II blockers can reduce the amount of microalbuminuria, but only ACE inhibitors have been shown to slow or halt the progression of renal disease. Therefore, ACE inhibitors should be the first line choice, and if intolerance develops to one drug, other ACE inhibitors should be tried first.
Ideally, screening microalbumin is best done on a first void morning urine (to avoid the effect of physical activity). If there is an albumin/creatinine ratio > 30 mcg/mg Cr, a urinalysis should be obtained to rule out pyuria or hematuria. These should be treated first. Tight glycemic control and treatment of hypertension, if present, should be initiated. Repeat urine microalbumin within 3 - 6 months is recommended before initiating ACE inhibitor treatment for confirmed microalbuminuria.
Data are still insufficient in this area to advise on titration of ACE inhibitor dosage in normotensive patients with microalbuminuria. The value of following microalbuminuria once therapy is established has not been determined in the literature. However, some experts believe that tight glycemic control and treatment of hypertension improve microalbumin; and, therefore following microalbuminuria once therapy has been established may be appropriate.
For additional information on nephropathy, see pages 33 - 34 in the Kaiser Permanente Northern California Clinical Practice Guidelines for the Management of Diabetes Mellitus, April 1998.
SELECTED REFERENCES GENERAL American Diabetes Association: Clinical Practice Recommendations 1999. Diabetes Care 1999;22(suppl l):SI-Sll4. Kaiser Permanente Care Management Institute. CMI Diabetes Guidelines 1999.
DIAGNOSIS/SCREENING Ehrmann DA, Bames RB, Rosenfield RL, et al. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care 1999:22:141-146.
Kilpatrick ES, Maylor PW, Keevil BG. Biological variation of glycated hemoglobin: implications for diabetes screening and monitoring. Diabetes Care 1998:21:261-264.
Peters AL, Davidson MB, Schriger DL, et al. A clinical approach for the diagnosis of diabetes mellitus: an analysis using glycosylated hemoglobin levels. JAMA 1996:276:1246-1252.
UK PROSPECTIVE DIABETES STUDY (UKPDS) TRIALS UK Prospective Diabetes Study (UKPDS) Group. Intensive blood glucose control with sulfonylureas or insulin compared with conventional treatment and risk of complications in patients with Type 2 diabetes (UKPDS 33).Lancet 1998;352:832-853.
UKPDS Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854-65.
UKPDS Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. Brit Med J. 1998;317:703-712.
UKPDS Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in tvpe 2 diabetes:UKPDS 38. Brit Med J. 1998;317:713-720.
UKPDS Group. Cost effectiveness analysis of improved blood pressure control in hypertensive patients with type 2 diabetes: UKPDS 40. Brit Med J. 1998;317:720-726.
ACE INHIBITORS The Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Effects of an angiotensin- converting-enzyme inhibitor, ramipril, on death from cardiovascular causes, myocardial infarction, and stroke in high-risk patients. NEngljMed 2000:342:145-53.
UPDATE ON TWO DRUGS MENTIONED IN GUIDEUNE: 1. Trogirtazone (Rezulin) has been withdrawn from the market (3/21/2000). 2. Cisapride (Propulsid) is available only through a limited-access program (7/14/2000).
HYPERTENSION Hansson L, Zanchetti A, Camithers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet 1998;351:1755-62.
LIPIDS Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFW\PS/TexCAPS.JAMA 1998;279:1615-22. Haffner S. Management of dyslipidemia in adults with diabetes. Diabetes Care 1998;21:160-78.
PNEUMOCOCCAL VACCINE American Diabetes Association: Clinical Practice Recommendations 2000. Diabetes Care 2000;23 (suppi 1):S91-S93. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP).MMWR 1997:46;1-24.
PERIPHERAL NEUROPATHY Backonja M, Beydoun A, Edwards KR et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial.JAMA 1998;250:1831-1836.
Morello CM, Leckband SG, Stoner CP et al. Randomized double-blind study comparing the efficacy of gabapentin with amitriptyline on diabetic peripheral neuropathv pain.Arch Intern Med 999;159:1931- 1937.
AUTONOMIC NEUROPATHY Koch KL. Diabetic gastropathy: gastric neuromuscular dysfunction in diabetes mellitus: a review of symptoms, pathophysiology, and treatment. Dig Dis Sci 1999;44:106l-75.
Kong MF. Horowitz M. Gastric emptying in diabetes mellitus; relationship to blood-glucose control. Clinical Geriatric Medicine 1999;15:321-38.
Patterson D, Abell T, Rothstein R, et al. A double-blind multicenter comparison of domperidone and metoclopramide in the treatment of diabetic patients with svmptoms of gastroparesis. Am J Gastroenterol 1999;94:12304.
SILDENAFIL Kloner RA,JarowJP Erectile dysfunction and sildenafil citrate and cardiologists..Amer J Cardiol 1999;83: 576-82.
Lipshultz LI, Kim ED. Treatment of erectile dysfunction in men with diabetes. JAMA 1999;281:465-6. Price DE, Gingell JC, Gepi-Attee S, et al. Sildenafil: study of a novel oral treatment for erectile dysfunction in diabetic men. Diabetic Medicine 1998;15:821-825.
Rendell MS, Rajifer J, Wicker PA, et al. Sildenafil for treatment of erectile dysflinction in men with diabetes: a randomized controlled trial. JAMA 1999;281:421-6.
ACKNOWLEDGMENTS Clinical Leader Barbara Livermore, MD; Endocrinology, Sacramento
Guideline Team Bill Caplan, MD, Endocrinology, Martinez Richard Kanter, MD, Endocrinology, San Francisco Jerry Minkoff, MD, Endocrinology, Santa Rosa
Project Management Julie Lenhart, RPh, MS; TPMG Department of Quality & Utilization David St. Pieire, MHROD; TPMG Department of Quality & Utilization
Data Analysis Ralph Vogel, PhD, TPMG Department of Quality and Utilization Patricia Kipnis, PhD, TPMG Department of Quality and Utilization
Reviewers Robert Alloo, MD; Medicine, Santa Clara/Campbell Antonis Antoniou, MD; Medicine, Fresno Tim Corfman, MD; Medicine, Walnut Creek John Tamor Citron; MD, Endocrinology, Walnut Creek Rick Diott. MD; Endocrinology, Martinez Laurie Doyle, MPH; Regional Health Education Bruce Ettinger, MD; Division of Research Paul Feigenbaum, MD; Medicine, San Francisco Robert Goldfien, MD; Medicine, Richmond Fred Horn, MD; Endocrinology, Fremont Mare Jaffe, MD; Endocrinology, South San Francisco Pamela Kershner, MD; Endocrinology, Walnut Creek Kevin Kobalter, MD; Endocrinology, San Rafael Pansy Kwong, MD; Medicine, Oakland Nancy Moline, RN, MEd, CDE; Regional Health Education Elia Racah, MD; Medicine, Park Shadelands Craig Sadur, MD; Endocrinology, Pleasanton Edgar Schoen, MD; Genetics/Pediatrics, Oakland Craig Smith, MD; Medicine, South Sacramento John Takakuwa, MD; Medicine, Rancho Cordova David Williams, MD; Medicine, Vallejo Jeannie Tip, MD; Medicine, Oakland
Editing & Graphic Design Linda Bine; TPMG Communications Gail Holan; Curvey Graphic Design
CONTACT INFORMATION Kaiser Permanente Northern California TPMG Department of Quality and Utilization 1800 Harrison Street, 4th Floor Oakland. CA 94612 510-987-2950 or tie-line 8-427-2950 To obtain more information about KPRC Clinical Practice Guidelines, printed copies. or permission to reproduce any portion, please contact the TPMG Dept. of Quality & Utilization, or send an e-mail message to clinical .guidelines@kp. org KPNC Clinical Practice Guidelines can be viewed on-line on the Kaiser Permanente Northern California intranet at http://cl.kp.org This website is accessible only from the Kaiser Permanente computer network.
Disclaimer The Permanente Medical Group (TPMG) Clinical Practice Guidelines have been developed to assist clinicians by providing an analytical framework for the evaluation and treatment of selected common problems encountered in patients. These guidelines are not intended to establish a protocol for all patients with a particular condition. While the guidelines provide one approach to evaluating a problem, clinical conditions may vary significantly from individual to individual. Therefore, the clinician must exercise independent judgment and make decisions based upon the situation presented. While great care has been taken to assure the accuracy of the information presented, the reader is advised that TPMG cannot be responsible for continued currency of the information, for any errors or omissions in this guidelines, or for any consequences arising from its use.
BACK to Kaiser Diagnostic and Treatment Documents Index